
Publicaciones
COVID-19 IMPORTS REPORT
Updated to June 30, 2020
Have any exemptions from standard customs procedures been introduced for products deemed necessary to combat the COVID-19 outbreak?
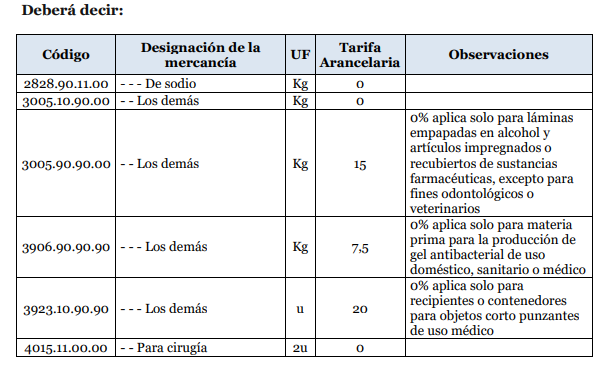
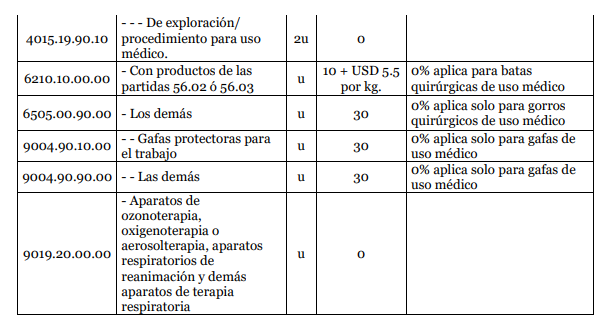
Yes[1]. The plenary session of the Foreign Trade Committee (COMEX) through Resolution 004-2020 established in Annex 1 the products that due to the sanitary emergency will have 0% of importation tariff rate.
What exemptions do these measures provide?
The importation of products and supplies that will help fight against the coronavirus pandemic will have 0% of importation tariff rate.
What products are covered by these measures?
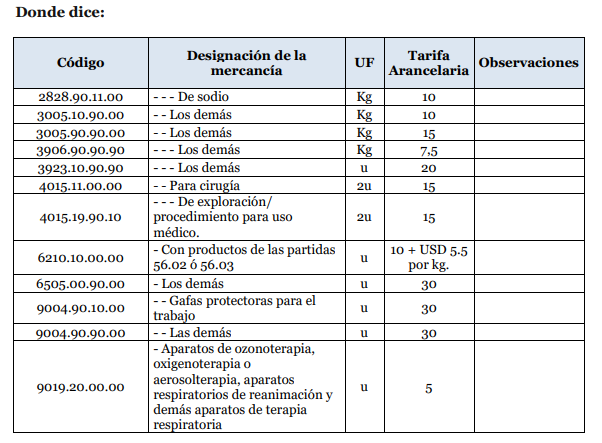
The National Customs Service of Ecuador, through bulletin 17-2020[2] has established the list of products, which are:
- Surgical caps
- Protective glasses for work
- Surgical masks
- Examination gloves
- Surgical scrubs
- COVID-19 rapid test – reagents
- Alcohol stamped sheet
- Disposable laryngoscope
- Ozone therapy, oxygen therapy or aerosol therapy devices
- Revival breathing devices and other respiratory therapy devices
- Catheter
- Needle for arterial / venous fistula
- Bandages
- Bacterial / viral hygroscopic filter
- Surgical gloves
- Container for Sharp objects
- Security glasses
- Disinfectant (sodium hypochlorite)
- Potassium monopersulfate
- Carbopol
- Quaternary ammonium disinfectant (with subheading 3808.94.19.00)
- Quaternary ammonium disinfectant (with subheading 3808.94.99.00)
- Filmarray PCR Rapid Test Breathing Panels
- Reagents (coronavirus)
Is there an expiration date for these measures?
This measure was taken according to the state of sanitary emergency that the country is going through, so it is expected that it will no longer be valid once the state of emergency has been overcome. Until now, the measures are maintained until August 14.
Have the customs authorities adopted any measures to assist traders more generally with current customs procedures?
Yes. The Ecuadorian Standardization Service – INEN, streamlines the import process of various medical supplies such as: resuscitation respiratory devices, respiratory therapy devices, raw material for the production of antibacterial gel for domestic, sanitary or medical use, protective glasses, masks, among others.
In this sense, INEN users must follow an easy and quick process, detailed below:
- Enter the Ecuapass system.
- Verify through the tariff subheadings whether or not the product requires an INEN Certificate of Recognition.
- Verify that the product is within the scope of the RTE (Technical Regulation).
- Evaluate the product in accordance with the corresponding RTE INEN technical regulation.
- It is evaluated as a product not subject to control with technical sheets and invoice.
- INEN approval is issued (INEN Recognition Certificate)
In case of not complying with any premise of the Technical Regulation, it is corrected so that the user makes the correction to his request. Immediately corrected, the INEN recognition certificate is issued.
It is important mention that users and / or importers must have complied with the corresponding customs procedures with the National Customs Service of Ecuador – SENAE to nationalize their merchandise[3].
Who can I contact for more information on these measures?
CONSULEGIS ABOGADOS
Jorge Vaca-Sánchez
[email protected]
ANNEX 1:
[1] https://www.aduana.gob.ec/boletines/senae-implementa-tarifa-cero/
[2]https://www.aduana.gob.ec/wp-content/uploads/2020/03/Listado-de-tarifas-arancelarias_Insumos-Productos-Salud-MSP.pdf
[3]https://www.normalizacion.gob.ec/inen-agiliza-el-proceso-de-importacion-de-insumos-medicos-para-centros-hospitalarios/
Sobre el autor